Jobs in Regulatory Affairs
Solid rules, excellent pharma, medical devices, ATMPs and cosmetics! QbD’s regulatory experts monitor everything, from strategy to approval and aftercare. Looking for interesting jobs in Regulatory Affairs (RA)? Explore your career options below and apply!
-
Consultant RA IVDRegulatory Affairs · QbD Flanders (HQ)
-
Senior Consultant RA IVDRegulatory Affairs · Multiple locations · Hybrid
-
Senior Consultant Regulatory AffairsRegulatory Affairs · QbD Spain (Madrid) · Hybrid
-
Consultant Legal Representative ServicesRegulatory Affairs · Multiple locations · Hybrid

What is Regulatory Affairs?
Regulatory Affairs acts as a liaison between the government, industry, and consumers to ensure products are safe and effective when used as intended.
Regulatory Affairs experts follow medical devices and pharmaceutical regulations worldwide – and all relevant standards – in order to support Research & Development teams in their work.
Moreover, Regulatory Affairs defines the optimal strategy to put a product on the market by collecting data and forming a dossier to be submitted for review.

Importance of Regulatory Affairs
Regulatory Affairs negotiates the interaction between all parties involved to get good products to the market quickly and prevent bad products from being sold.
On the one hand, Regulatory Affairs looks after the interests of life science companies, on the other, they protect public health.

Jobs and career paths in Regulatory Affairs
Regulatory Affairs Specialist is a multidisciplinary, cross-functional position, in which you define the manufacturer’s Regulatory Affairs strategy, link all departments, and follow the full lifecycle of the product, from idea to end. So good networking skills and curiosity are crucial!
Do you have a Master’s or a PhD in Sciences, Engineering, Pharmaceuticals, or Biomedical Sciences? An interest in regulation, good practices, and quality? And do you want to support the launch of new medical devices or pharmaceutical products? Then Regulatory Affairs is the perfect career path for you.
Choose your own career path!
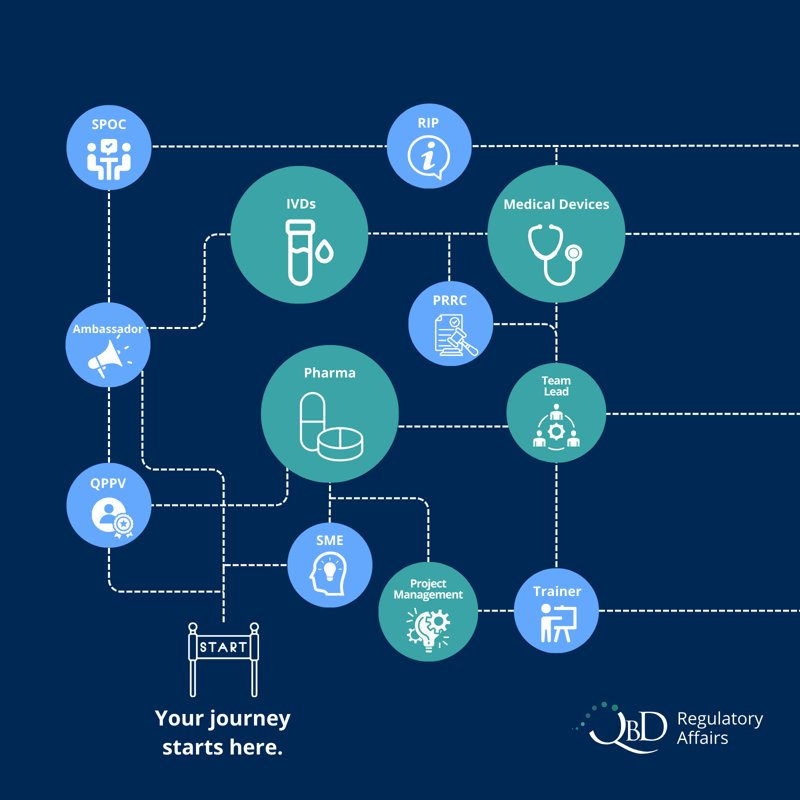
-
The role of the Regulatory Affairs professional involves interactions with many other departments and functions. Working in the Regulatory Affairs Division at QbD Group offers the chance to be part of an international team, interacting with colleagues at different QbD Group offices to deliver successful projects in collaboration with a diverse range of global clients. Whether working with a client we have partnered with for over a decade, or starting on a new project with a new client, you expect a different challenge each day! Coupled with the fact that the regulations and guidelines that underpin our role are frequently updated, this presents the opportunity for continued learning, personal development and sharing information with colleagues across QbD Group and our wider networks. Having begun my own career in drug discovery roles before moving into regulatory affairs, it is rewarding to contribute to client projects that achieve milestones in clinical development, registration or commercialisation, as I really understand how much time went into their initial discovery!
Peter Fry
Business Unit Manager, Regulatory Affairs Pharma & Country Manager, UK
Connect with our expert recruiters
Our team of specialized recruiters is here to help you take the next step in your career. Below, you'll find the experts focused on this service area, ready to assist you in finding the perfect opportunity. Reach out to them directly for personalized guidance and support throughout your job search.



